Mitochondrial dynamics in cell physiology and disease
Overview
The primary focus of our lab is to understand the role of mitochondrial dynamics in normal cellular function and human disease. Due to their well-known role in oxidative phosphorylation, mitochondria are commonly thought of as the "powerhouses" of the cell. However, they are also involved in many other cellular functions, including fatty acid oxidation, iron-sulfur metabolism, programmed cell death, calcium handling, and innate immunity. They are remarkably dynamic organelles that undergo continual cycles of fusion and fission, events that result in mixing of mitochondrial contents. The equilibrium of these two opposing processes determines the overall morphology of mitochondria and has important consequences for the quality of the mitochondrial population.
Our research falls into several broad areas:
(1) What are the cellular and physiological functions of mitochondrial fusion and fission?
(2) What is the molecular mechanism of mitochondrial membrane fusion and fission?
(3) What role do mitochondrial dynamics play in human diseases?
To address these issues, we use a wide range of approaches, including genetics, biochemistry, cell biology, and structural biology.
Cellular and physiological functions of mitochondrial dynamics
A typical mammalian cell can have hundreds of mitochondria. However, each mitochondrion is not autonomous, because fusion and fission events mix mitochondrial membranes and contents. As a result, such events have major implications for the function of the mitochondrial population. We are interested in understanding the cellular role of mitochondrial dynamics, and how changes in mitochondrial dynamics can affect the function of vertebrate tissues.

We have used mouse genetics to determine the physiological functions of mitochondrial dynamics. One part of our work focuses on proteins called mitofusins (Mfn1 and Mfn2), which are transmembrane GTPases embedded in the outer membrane of mitochondria. These proteins are essential for fusion of mitochondria. To understand the role of mitochondrial fusion in vertebrates, we have constructed mice deficient in either Mfn1 or Mfn2. We find that mice deficient in either Mfn1 or Mfn2 die in mid-gestation due to placental insufficiency. Mfn2 mutant embryos have a specific and severe disruption of a layer of the placenta called the trophoblast giant cell layer. These findings indicate that mitochondrial fusion is essential for embryonic development and that specific cell types can show high vulnerability to reduced mitochondrial fusion. We have also utilized conditional alleles of Mfn1 and Mfn2 to examine the role of mitochondrial fusion in adult tissues such as the cerebellum, skeletal muscle, heart, and the substantia nigra. These studies are relevant to our understanding of several human diseases (see below). Mice deficient in mitochondrial fission also have severe tissue defects. Remarkably, we find that the equilibrium between the rates of fusion and fission is key, rather than the absolute rates of fusion or fission. Mice deficient in either Mff (mitochondrial fission factor) or Mfn1 have lethal phenotypes; however, mice deficient in both genes are healthy.
Embryonic fibroblasts lacking Mfn1 or Mfn2 display fragmented mitochondria, a phenotype due to a severe reduction in mitochondrial fusion. Cells lacking both Mfn1 and Mfn2 have completely fragmented mitochondria and show no detectable mitochondrial fusion activity. Our analysis indicates that mitochondrial fusion is important not only for maintenance of mitochondrial morphology, but also for cell growth, mitochondrial membrane potential, maintenance of the mitochondrial genome, and cellular respiration. These studies indicate that mitochondrial dynamics serves to maintain mitochondrial function by homogenizing the mitochondrial population through content exchange.
Beyond fusion and fission, another aspect of mitochondrial dynamics is the selective degradation of aged or dysfunctional mitochondria. The major pathway for mitochondrial degradation is mitophagy, in which defective mitochondria are recognized, segregated, and removed through autophagy. We are studying pathways that mediate mitochondrial quality control through mitophagy. It is thought that some diseases, such as familial Parkinson's disease, may arise through defects in the removal of defective mitochondria.


Molecular mechanism of membrane fusion and fission
The best understood membrane fusion proteins are viral envelope proteins and SNARE complexes. Viral envelope proteins, such as gp41 of HIV, reside on the lipid surface of viruses and mediate fusion between the viral and cellular membranes during virus entry. SNARE complexes mediate a wide range of membrane fusion events between cellular membranes. In both cases, cellular and crystallographic studies have shown that the formation of helical bundles plays a critical role in bringing the merging membrane together. We would like to understand mitochondrial fusion at a similar level of resolution and to determine whether there are common features to these diverse forms of membrane fusion.
Mitofusins are the only conserved mitochondrial outer membrane proteins involved in fusion. Therefore, it is likely that they directly mediate membrane fusion. Consistent with this idea, mitofusins are required on adjacent mitochondria to mediate fusion. In addition, mitofusins form homotypic and heterotypic complexes that are capable of tethering mitochondria. We are trying to determine how tethered mitochondria, mediated by mitofusins, proceeds to full fusion. Mitochondrial fusion is likely to be more complicated than most other intracellular membrane fusion events, because four lipid bilayers must be coordinately fused. Whereas mitofusins mediate outer membrane fusion, OPA1, another large GTPase, mediates inner membrane fusion. We are studying how the fusion activity of OPA1 is controlled.
Mitochondrial fission is mediated by the dynamin-related GTPase Drp1. A pool of Drp1 resides in the cytosol and is recruited to the mitochondrial surface by receptor molecules on the mitochondrial outer membrane. We have solved crystal structures of Drp1 receptors in both yeast and mammalian systems. These studies will reveal how these receptors regulate the recruitment of Drp1 for mitochondrial fission.
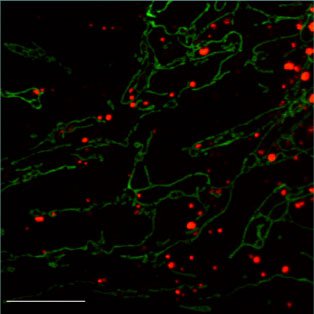

Mitochondrial dynamics in human disease
Mitochondrial dynamics is important for human health. Two inherited human diseases are caused by defects in mitochondrial fusion. Charcot-Marie-Tooth (CMT) disease is a neurological disorder that affects the peripheral nerves. Patients with CMT experience progressive weakness of the distal limbs and some loss of sensation. A specific type of CMT, termed CMT2A, is caused by mutations in Mfn2 and result from degeneration of axons in peripheral nerves. We have analyzed the functional consequences of such disease alleles, and have used transgenic and targeted mutagenesis approaches to develop mouse models. The most common inherited form of optic neuropathy (autosomal dominant optic atrophy) is caused by mutations in OPA1. This mitochondrial protein is localized to the inner membrane space and is essential for mitochondrial fusion. We have analyzed how disease alleles affect the function of OPA1, particularly its GTP hydrolysis and lipid membrane deforming activities. Defects in mitochondrial fission also cause severe human diseases. Mutations in the mitochondrial fission factors Drp1 or Mff cause a wide range of neurological defects.
Finally, an understanding of mitochondrial dynamics will be essential for understanding a large collection of diseases termed mitochondrial encephalomyopathies. Many mitochondrial encephalomyopathies result from mutations in mitochondrial DNA (mtDNA). In mtDNA diseases, tissues maintain their mitochondrial function until pathogenic mtDNA levels exceed a critical threshold. Experiments with cell hybrids indicate that mitochondrial fusion, by enabling cooperation between mitochondria, can protect respiration even when >50% of mtDNAs are mutant. To understand the pathogenesis of mtDNA diseases, it is critical to explore how mitochondria can be functionally distinct and yet cooperate as a population within a cell. We anticipate that our studies with mice lacking mitochondrial fusion will help to shed light on this group of often devastating diseases.
